Chromosome 10qter Deletion Syndrome a Review and Report of Three New Cases
- Case report
- Open Access
- Published:
Clinical, cytogenetic and molecular report of a case of ring chromosome 10
Molecular Cytogenetics volume 8, Article number:29 (2015) Cite this article
Abstract
Ring chromosome 10 is a rare cytogenetic finding. Only a few cases with molecular cytogenetic definition have been reported. We written report here on a kid with a ring chromosome ten, which is associated with prenatal and postnatal growth retardation, microcephaly, dysmorphic features, hypotonia, heart defect, severe pes equinovarus, and bronchial asthma. The chromosomal aberration was defined by chromosome microarray analysis, which revealed two deletions at 10pter (3.68 Mb) and 10qter (4.26 Mb). The clinical features are very like to those reported in other clinical cases with band chromosome 10, excluding bronchial asthma, which has not been previously reported in individuals with ring chromosome 10.
Background
Ramble band chromosomes have been identified for each of the man chromosomes, and overall frequency is estimated at 1 in 30,000 to 60,000 births [1]. Rings result from rare intrachromosomal fusions, although the mechanisms underlying chromosomal ring formation are not completely understood.
Band chromosome ten is a rare cytogenetic finding, currently reported in 17 unrelated patients. Common clinical features in these patients include brusk stature, intellectual inability, microcephaly, facial dysmorphism, and ophthalmologic and urinary tract abnormalities [two]. Clinical features vary, even so, depending on the position of the breakpoints and on the level of mosaicism resulting from the unstable nature of the ring upon cell sectionalisation [3]. Thus, a comprehensive diagnosis of an individual with a ring chromosome requires both a molecular diagnostic approach such as array-CGH and a cytogenetic approach to determine a specific individual diagnosis. Here we draw the clinical features of the patient with the largest apparently stable ring chromosome x.
Example presentation
The patient is a 13-month-old daughter born at 36 weeks of gestation to not-consanguineous and healthy Caucasian parents aged 27 years (mother) and 33 years (father). In utero, intra-uterine growth retardation with meconium staining in the amniotic fluid was observed. She was delivered by elective caesarean section. At nativity her weight was ane,600 yard (−2.5 SD), her length was 40 cm (-2.5 SD), and the head circumference was thirty cm (−1 SD). Apgar scores were iv–8. Severe congenital pes equinovarus was detected from birth. She was also noted to have hypotonia. Presently after birth, treatment with the Ponseti method was started with surgical correction of the Achilles tendon at 3 months of historic period. Neurosonoscopy revealed balmy widening of the ventricles, but re-evaluation after 1 calendar month was normal. She was found to have normal hearing acuity afterward birth. A cardiac ultrasound test showed a large patent ductus arteriosus. She had no feeding difficulties. Abdominal organs were without structural abnormalities. At the historic period of 4 months, bronchial asthma was diagnosed and she has been receiving medical treatment e'er since. Examination at the age of 7 months revealed delayed speech and gross motor skills. Her height, weight, and head circumference were significantly less than the 3rd percentile. Furthermore, the patient had dysmorphic features consisting of microcephaly, slight metopic ridge, low-set ears, downslanting and narrowing of palpebral fissures, wide nasal bridge, stubby nose, smoothen philtrum with sparse upper lip and everted lower lip, microstomia, narrow palate, curt neck, inverted and widely-spaced nipples, broad hands, tapering fingers, unmarried palmar crease on the left palm, and broad anxiety with brusque toes and small nails (Figure i). Mild divergent strabismus was documented at that time. Otolaryngological evaluations revealed a deviated septum. Skull roentgenograms revealed no synostosis.
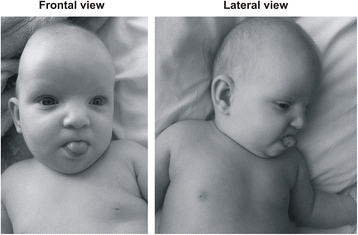
Photographs of the patient at the age of seven months (frontal and lateral view). Note the downslanting and narrowing of palpebral fissures, broad nasal bridge, stubby nose, shine philtrum with thin upper lip and everted lower lip, microstomia, low-gear up ears, and curt neck.
On the terminal examination at 13 months of historic period, her development milestones were establish to be delayed. She could non sit unsupported and her head control was insufficient. She showed practiced visual fixation. There was no speech evolution. Muscle tone was decreased and deep tendon reflexes were normal. She displayed unusual repetitive manus movements, continuously pressing her palms together in the midline and repetitively stroking her thumbs. A brain MRI was declined by her parents. At the age of 7 months, the proband was referred to a clinical geneticist.
Materials and methods
Standard cytogenetics
Cultures of the patient's peripheral blood were established and harvested according to standard laboratory protocols. Chromosome preparations were treated with trypsin and stained with Giemsa. A full of 30 metaphase cells were analysed at the 550-band resolution level. The karyotypes were described according to the guidelines of the International Organisation for Human Cytogenetic Nomenclature [46,20,r(10)(p15.1q26.1)]. Additionally, 370 cells were counted to verify band instability. The parents declined to undergo chromosomal analysis.
Molecular cytogenetics
Dna was extracted from the patient'south peripheral white blood cells using the phenol-chloroform extraction method. A subsequent array-comparative genomic hybridisation (array-CGH) test was performed to determine the chromosomal breakpoints of the ring, too equally other possible chromosomal abnormalities that may have been missed past routine One thousand-banded chromosomal assay. Agilent Homo ISCA CGH 180 K microarrays with an average spatial resolution of 25 kb were used in the study (Agilent Technologies, Santa Clara, CA). Genomic DNA from the proband and pooled normal male reference DNA (Agilent Technologies) were digested with Covaris S220 (Life Technologies, Grand Island, NY) and labelled with an Agilent Genomic DNA labelling kit co-ordinate to the manufacturer'due south recommendations. Patient and reference DNA were labelled with Cy5 and Cy3 respectively and were co-hybridised to arrays for 24 h at 67°C in a rotating oven (Agilent technologies) at twenty rpm. The arrays were then washed and scanned with an Agilent Microarray Scanner. Data were extracted using Feature Extraction x.vii.ane software (Agilent Technologies) and analysed using Cytogenomics two.ix.two.4 software (Agilent Technologies). Genomic copy number changes were identified with the assist of the Aberration Detection Method ii algorithm with the sensitivity threshold set at 6.0. Copy number changes identified in the samples were evaluated by using the UCSC Genome Browser website (http://genome.ucsc.edu) and the Database of Genomic Variants (http://projects.tcag.ca/variation). The array information was analysed using notation GRCh37/hg19. The DECIPHER (http://decipher.sanger.ac.uk/) database was used to back up genotype-phenotype correlation.
Results
Cytogenetic assay revealed an apparently stable not-mosaic ring chromosome 10 [46,XX,r(10) (p15.1q26.one)]. Secondary aberrations (ii separate rings and interlocked rings) were found in less than 5% of the mitoses counted, 1.seven% and 0.5% respectively. High-resolution breakpoint mapping with a Human ISCA CGH 180 One thousand microarray re-defined the karyotype as 46,XX,r(ten)(p15.2q26.3).arr[hg19]10p15.3p15.2(1–3,678,763)×1,10 q26.3(131,276,836–135,534,747)×1, indicating an approximately 3.68 Mb deletion in 10p and a 4.26 Mb deletion in 10q (Figure 2). No other relevant genomic imbalance was establish.
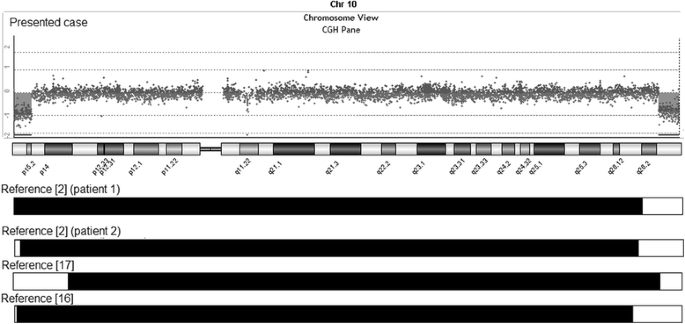
Chromosome 10 array-CGH profile of the patient showing a iii.68 Mb deletion at 10pter and a 4.26 Mb deletion at 10qter. A comparison of the extension of the deletions with previously reported patients with ring chromosome 10 is too shown (white bars).
Word
Ring chromosome 10 is a rare disorder. Only seventeen cases of band chromosome 10 have been reported in literature and more often than not defined by G banding [iii-15] and simply four cases with molecular cytogenetic definition [2,sixteen,17]. This is the fifth instance with precisely defined r(x) helping to meliorate establish a karyotype-phenotype correlation.
Nosotros compared the phenotype and genotype of our patient with previously published patients having precisely defined breakpoints [2,xvi,17]. The patient we present has the largest ring chromosome 10 reported to engagement. In all previously reported patients with ring chromosome 10, the breakpoints in 10q are variable only more proximal to the centromere (Figure 2).
Common clinical features in patients with ring chromosome x include prenatal and postnatal growth retardation, varying degrees of intellectual disability, microcephaly, and dysmorphic features (wide nasal bridge, strabismus, hypertelorism, low-set malformed ears) [xviii,xix] that are non-specific to distal 10q deletion and are mutual to many chromosome anomalies. It is unlikely that iii.68 Mb final 10p15.two deletion in the present ring chromosome or smaller deletion in instance of band chromosome reported by Gunnarson et al. would make a meaning contribution to the phenotype. However the patient with a larger deletion of chromosome 10 brusk arm has boosted clinical features such as talipes equinovarus, hepatomegaly, splenomegaly that crusade severe phenotype [17].
Nosotros also compared the clinical features of our patient with patients from DECIPHER (Tabular array one). Cases with complex chromosomal rearrangements or with larger deletions than those identified in our patient and cases without a detailed clinical description were excluded from the comparison. Based on comparison of the clinical features of our patient with the clinical features of the patients with ring chromosome and the clinical features of patients with pure terminal deletions of 10p and 10q, the contribution of the terminal deletion 10q to the clinical phenotype of our patient is the most significant.
The study of patients with a distal pure 10q deletion has revealed the existence of a minimal disquisitional region (MCR), which was recently assigned past Yatsenko et al. [20] to an approximately 600 kb segment in the distal part of chromosome 10, which encompasses two annotated genes, C10ORF90 (chromosome 10 open up reading frame 90) and DOCK1 (dedicator of cytokinesis 1). Nosotros predict that the overlapping phenotype of pure 10q deletions at 10q26.two region could exist caused past haploinsufficiency of i or more genes or position effect, since 10q26.3 deletion detected in our patient does non involve these genes.
The deleted region 10q26.iii, 4.26 Mb in size, contains 31 poly peptide coding genes of which PPP2R2D (poly peptide phosphatase 2, regulatory subunit B, delta), JAKMIP3(Janus kinase and microtubule interacting protein 3), DPYSL4 (dihydropyrimidinase-like 4), INPP5A (inositol polyphosphate-5-phosphatase), GPR123 (Grand protein-coupled receptor 123), GLRX3 (glutaredoxin 3) and ADAM8 (ADAM metallopeptidase domain 8) could be considered of import contributors to the clinical phenotype. Based upon function and high expression in the brain [http://www.proteinatlas.org/], nosotros advise that the haploinsuffiency of PPP2R2D, JAKMIP3, DPYSL4, and GPR123 could play significant roles in neurodevelopmental filibuster. PPP2R2D is essential for many betoken transduction pathways [21]. JAKMIP3 is associated with caveolin-1, which recruits synaptic components and regulates the betoken transduction of a variety of neurotransmitter and neurotrophic receptors in the central nervous system (CNS) [22]. The collapsin response mediator protein encoded by DPYSL4 is thought to be involved in semathorin-induced growth cone collapse during neural development. Down-regulation of DPYSL4 expression using siRNA shows an early increase in neurite outgrowth, further supporting the idea that DPYSL4 inhibits microtubule polymerisation and neurite outgrowth [23]. The CNS-specific expression of GPR123, together with the high sequence conservation between the vertebrate sequences investigated, betoken that GPR123 may have an important office in the regulation of neuronal bespeak transduction [24].
Craniofacial dysmorphisms, foot abnormalities, and brusk stature could be attributed to the loss of the GLRX3 cistron. Although growth delay is usually associated with the band chromosome of whatsoever autosome, perhaps due to ring instability [1], stature might too correlate with the haploinsuffiency of genes that encode protein and play a role in cell growth. The ubiquitous expression of Glrx3 in mouse embryos and tissues indicates that Glrx3 is required for cell growth, organ evolution, and normal metabolism during growth and development [25]. Thus, deletion of GLRX3 might influence the severity of growth delay. The patent ductus arteriosus could be associated with the haploinsuffiency of DPYSL4, PPP2R2D, and INPP5A, the expression of which is predominant in the heart [http://www.proteinatlas.org/].
The 2d deleted region, 10p15.two-pter, could also contribute to the observed phenotype. The 10p15.3p15.two deleted region contains 11 protein-coding genes (TUBB8 (tubulin, beta 8 class Viii), ZMYND11 (zinc finger, MYND-type containing eleven), DIP2C (DIP2 disco-interacting protein 2 homolog C), PRR26 (proline rich 26), LARP4B (La ribonucleoprotein domain family, member 4B), GTPBP4 (GTP binding protein 4), IDI2 (isopentenyl-diphosphate delta isomerase 2), WDR37 (WD echo domain 37), ADARB2-AS1 (ADARB2 antisense RNA 1), PFKP (phosphofructokinase, platelet), PITRM1 (pitrilysin metallopeptidase 1), from which DIP2C and ZMYND11 could exist considered important contributors to growth delay, since they were virtually commonly deleted in DECIPHER patients with mutual clinical features, short stature and microcephaly (Table 1). ZMYND11 [26] and DIP2C [27] are expressed in various tissues, including the encephalon, simply piddling is known about their function. Gunnarson et al. stated that loss of 10p15.3 region including ZMYND11 would contribute little to the clinical phenotype considering of significant larger terminal deletion at 10q [16]. However ZMYND11 was suggested by DeScipio et al. as a principal contributor to the clinical features associated with 10p15 deletions based on genotype-phenotype of the cases with isolated 10p deletions [28].
In addition to the clinical features commonly found in patients with ring chromosome, bronchial asthma was present in our patient. This clinical characteristic had not previously been reported in patients with band chromosome 10. The ADAM8 mapped at 10q26 could exist involved in asthma pathogenesis. In humans, ADAM8 is expressed by most leukocytes [29,30], lung epithelial cells [31], and osteoclasts [32]. More recently, ADAM8 has been strongly associated with allergic airway inflammation (AAI) in humans and mice, and additional studies of ADAM8 are start to shed light on its roles in asthma pathogenesis [33].
Conclusions
The instance reported hither together with clinical and molecular findings, compared to previously published cases, highlights the importance of microarray analysis for patients with ring chromosomes, since it helps to delineate specific phenotypes. Nosotros were able to determine the gene content of the regions and make karyotype-phenotype correlations after having refined the verbal breakpoints of the deletions. Further functional studies of candidate genes are needed to prove biological significance in growth and development.
Consent
This case report is presented with the informed consent of the patient's parents. A copy of the written consent is available for review past the editor-in-primary of this journal.
Abbreviations
- Array-CGH:
-
Assortment based comparative genomic hybridisation
- CNS:
-
Primal nervous organization
References
-
Kosztolányi Chiliad. The genetics and clinical characteristics of constitutional ring chromosomes. J Assoc Genet Technol. 2009;35(two):44–eight.
-
Guilherme RS, Kim CA, Alonso LG, Honjo RS, Meloni VA, Christofolini DM, et al. Ring chromosome ten: report on 2 patients and review of the literature. J Appl Genet. 2013;54(one):35–41.
-
Nakai H, Adachi M, Katsushima N, Yamazaki N, Sakamoto M, Tada K. Ring chromosome 10 and its clinical features. J Med Genet. 1983;xx:142–iv.
-
Lansky S, Daniel W, Fleizar K. Physical retardation associated with band chromosome mosaicism: 46, 20, r(10)/45, 20,-ten. J Med Genet. 1977;14:61–three.
-
Fryns P, De Boeck M, Jaken J, van den Berg H. Malformative syndrome associated with a ring 10 chromosome and translocated 10q/nineteen chromosome. Hum Genet. 1978;43:239–44.
-
Sparkes RS, Ling SM, Muller H. Ring x chromosome: 46, XX, r(10)(p15q26). Hum Genet. 1978;43:341–v.
-
Simoni Chiliad, Rossella F, Dalpra 50, Visconi G, Piria-Schwaz C. Ring chromosome 10 associated with multiple congenital malformations. Hum Genet. 1979;51:117–21.
-
Tsukino R, Tsuda Northward, Dezawa T, Ishii T, Koike M. Ring chromosome 10: 46, XX, r(10)(p15q26). J Med Genet. 1980;17:148–50.
-
Michels VV, Driscoll DJ, Ledbetter DH, Riccardi VM. Phenotype associated with ring 10 chromosome: report of patient and review of literature. Am J Med Genet. 1981;ix:231–7.
-
Serville F, Briault R, Taillemite JL, Despoisse S, Cotoni P, Broustet A. Band chromosome x: 46, XX, r(ten)(p15q26). Ann Genet. 1982;25:168–71.
-
Kondo I, Shimakura Y, Hirano T, Kaneko M, Yabuta K. Ring chromosome ten syndrome: case report and the possibility of clinical diagnosis. Clin Genet. 1984;25:196–200.
-
Kishi Chiliad, Ikeuchi T, Yamamoto G, Tonomura A, Sakurada Northward, Satoh Y. Report of a patient with a ring chromosome ten: mos45, XY,-10/46, XY/46, XY, r(10)(p15.3q26.iii). Jinrui Idengaku Zasshi. 1985;30:233–8.
-
Higashi 1000, Sarashina N, Okamoto T, Matsuki C, Heim S. Supernumerary ring marker chromosome every bit a secondary rearrangement in a parapharyngeal lipoma with t(ten;12)(q25;q15) as the chief karyotypic aberration. Cancer Genet Cytogenet. 1992;64:163–v.
-
Calabrese G, Franchi PG, Stuppia L, Mingarelli R, Rossi C, Ramenghi L, et al. A newborn with band chromosome 10, aganglionic megacolon, and renal hypoplasia. J Med Genet. 1994;31:804–half-dozen.
-
Concolino D, Iembo MA, Moricca MT, Strisciuglio P, Marotta R, Rossi E, et al. Ring chromosome 10 (p15q26) in a patient with unipolar affective disorder, multiple minor anomalies, and mental retardation. Am J Med Genet. 2003;123A:201–3.
-
Gunnarsson C, Graffmann B, Jonasson J. Chromosome r(x)(p15.3q26.12) in a newborn kid: instance report. Mol Cytogenet. 2009;ii:1–6.
-
Christopoulou G, Tzetis K, Konstantinidou AE, Tsezou A, Kanavakis E, Kitsiou-Tzeli S, et al. Clinical and molecular description of a fetus in prenatal diagnosis with a rare de novo ring 10 and deletions of 12.59 Mb in 10p15.three–p14 and 4.22 Mb in 10q26.iii. Eur J Med Genet. 2012;55:75–9.
-
Wulfsberg EA, Weaver RP, Cunniff CM, Jones MC, Jones KL. Chromosome 10qter deletion syndrome: A review and report of three new cases. Am J Med Genet. 1989;32:364–7.
-
Plaisancie J, Bouneau L, Cances C, Garnier C, Benesteau J, Leonard Due south, et al. Distal 10q monosomy: New evidence for a neurobehavioral condition? Eur J Med Genet. 2014;57:47–53.
-
Yatsenko SA, Kruer MC, Bader PI, Corzo D, Schuette J, Keegan CE, et al. Identification of critical regions for clinical features of distal 10q deletion sindrome. Clin Genet. 2009;76:54–62.
-
Batut J, Schmierer B, Cao J, Raftery LA, Hill CS, Howell M. Two highly related regulatory subunits of PP2A exert opposite effects on TGF-beta/Activin/Nodal signalling. Development. 2008;135(17):2927–37.
-
Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell Mol Life Sci. 2010;67:3785–95.
-
Aylsworth A, Jiang SX, Desbois A, Hou ST. Characterization of the role of total-length CRMP3 and its calpain-broken product in inhibiting microtubule polymerization and neurite outgrowth. Exp Cell Res. 2009;315(sixteen):2856–68.
-
Lagerström MC, Rabe N, Haitina T, Kalnina I, Hellström AR, Klovins J, et al. The evolutionary history and tissue mapping of GPR123: specific CNS expression pattern predominantly in thalamic nuclei and regions containing large pyramidal cells. J Neurochem. 2007;100(4):1129–42.
-
Cheng NH, Zhang W, Chen WQ, Jin J, Cui 10, Butte NF, et al. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J. 2011;278(14):2525–39.
-
Kurozumi K, Nishita M, Yamaguchi 1000, Fujita T, Ueno North, Shibuya H. BRAM1, a BMP receptorassociated molecule involved in BMP signaling. Genes Cells. 1998;3:257–64.
-
Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, et al. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. Dna Res. 1999;6:63–70.
-
DeScipio C, Conlin Fifty, Rosenfeld J, Tepperberg J, Pasion R, Patel A, et al. Subtelomeric deletion of chromosome 10p15.3: Clinical findings and molecular cytogenetic characterization. Am J Med Genet A. 2012;158A:2152–61.
-
Richens J, Fairclough 50, Ghaemmaghami AM, Mahdavi J, Shakib F, Sewell HF. The detection of ADAM8 poly peptide on cells of the man allowed organisation and the demonstration of its expression on peripheral blood B cells, dendritic cells and monocyte subsets. Immunobiology. 2007;212:29–38.
-
Gomez-Gaviro Thousand, Dominguez-Luis M, Canchado J, Calafat J, Janssen H, Lara-Pezzi Eastward, et al. Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on 50-selectin shedding. J Immunol. 2007;178:8053–63.
-
Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007;119:863–71.
-
Ainola One thousand, Li TF, Mandelin J, Hukkanen M, Choi SJ, Salo J, et al. Involvement of ADAM8 in osteoclastogenesis and pathological bone destruction. Ann Rheum Dis. 2008;68:427–34.
-
Knolle MD, Owen CA. ADAM8: A new therapeutic target for asthma. Expert Opin Ther Targets. 2009;13:523–40.
Acknowledgements
Nosotros are grateful to the patient's family unit for participating in this study and for granting permission to publish photographs of the patient.
This work was partly supported by project "Promotion of Pupil Scientific Activities" (VP1-3.i-ŠMM-01-V-02-003) from the Research Council of Republic of lithuania (DV). This project is funded by the Republic of Lithuania and European Social Fund under the 2007–2013 Man Resources Development Operational Programme's priority 3.
Author information
Affiliations
Corresponding author
Boosted information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ŽČ and DV performed the assortment-CGH assay, interpreted the assortment-CGH data, drafted the manuscript, and contributed as. BB gathered clinical information and drafted the manuscript. ED carried out the cytogenetic evaluation. AU and VK revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript
Authors' data
ŽČ: Postdoctoral Fellow, Department of Homo and Medical Genetics, Vilnius University, Lithuania. Medical Geneticist, Middle for Medical Genetics, Vilnius University Infirmary Santariškių Klinikos, Republic of lithuania. BB: PhD Candidate, Section of Human and Medical Genetics, Vilnius Academy, Republic of lithuania. Clinical Geneticist, Centre for Medical Genetics, Vilnius University Infirmary Santariskių Klinikos, Lithuania. DV: Bachelor Student, Faculty of Natural Sciences, Vilnius University. ED: Clinical Geneticist, Centre for Medical Genetics, Vilnius University Infirmary Santariškių Klinikos, Lithuania. VK: Professor and principal investigator, Section of Human and Medical Genetics. Consultant, Centre for Medical Genetics, Vilnius University Hospital Santariškių Klinikos, Lithuania. AU: Managing director, Centre for Medical Genetics, Vilnius Academy Hospital Santariškių Klinikos, Lithuania. Professor, Section of Human and Medical Genetics, Vilnius University, Lithuania.
Co-start authors: Živilė Čiuladaitė and Birutė Burnytė.
Rights and permissions
This is an Open Admission article distributed nether the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/past/iv.0), which permits unrestricted apply, distribution, and reproduction in any medium, provided the original piece of work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/i.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
Virtually this article
Cite this article
Čiuladaitė, Ž., Burnytė, B., Vansevičiūtė, D. et al. Clinical, cytogenetic and molecular study of a case of ring chromosome 10. Mol Cytogenet 8, 29 (2015). https://doi.org/ten.1186/s13039-015-0124-9
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13039-015-0124-9
Keywords
- Ring chromosome x
- Dysmorphic features
- Bronchial asthma
- Array-CGH
Source: https://molecularcytogenetics.biomedcentral.com/articles/10.1186/s13039-015-0124-9
0 Response to "Chromosome 10qter Deletion Syndrome a Review and Report of Three New Cases"
Post a Comment